Costacurta specialises in mechanical engineering and manufactures radial flow reactor internals used in the chemical sector to produce ammonia.
Ammonia is one the most important inorganic chemical products and is used in many industries, above all in the production of fertilisers and to produce other sub-products such as urea, ammonium nitrate and ammonium sulphate and phosphate. Ammonia is also the precursor for many other chemicals such as nitric acid, hydrazine, acrylonitrile and hexamethylenediamine.
Ammonia is generally obtained from natural gas (ch4), which is chemically treated to obtain hydrogen which, reacting with the nitrogen in a synthesis reactor, produces ammonia (nh3).
Do you have a specific request?
Contact us now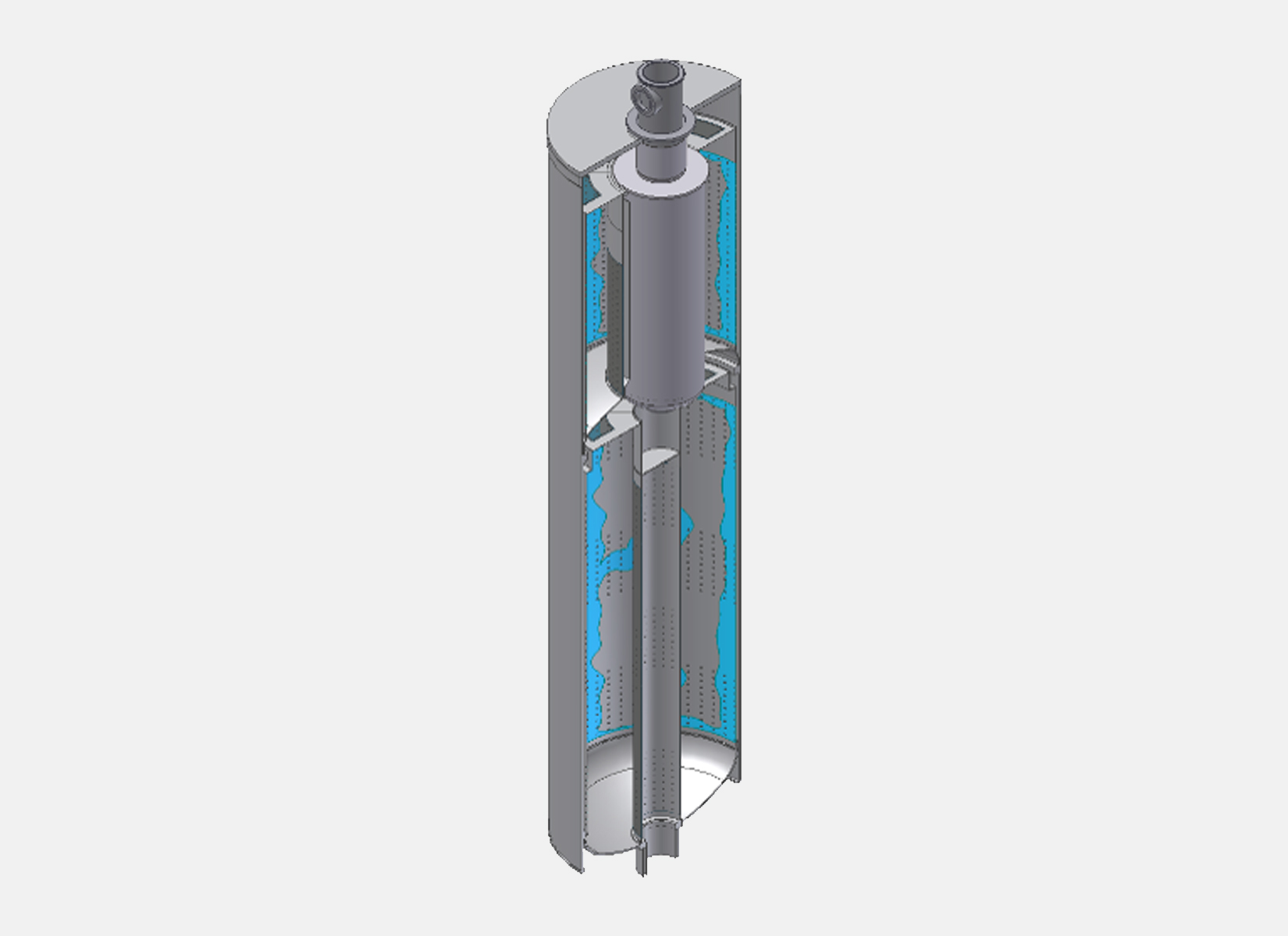
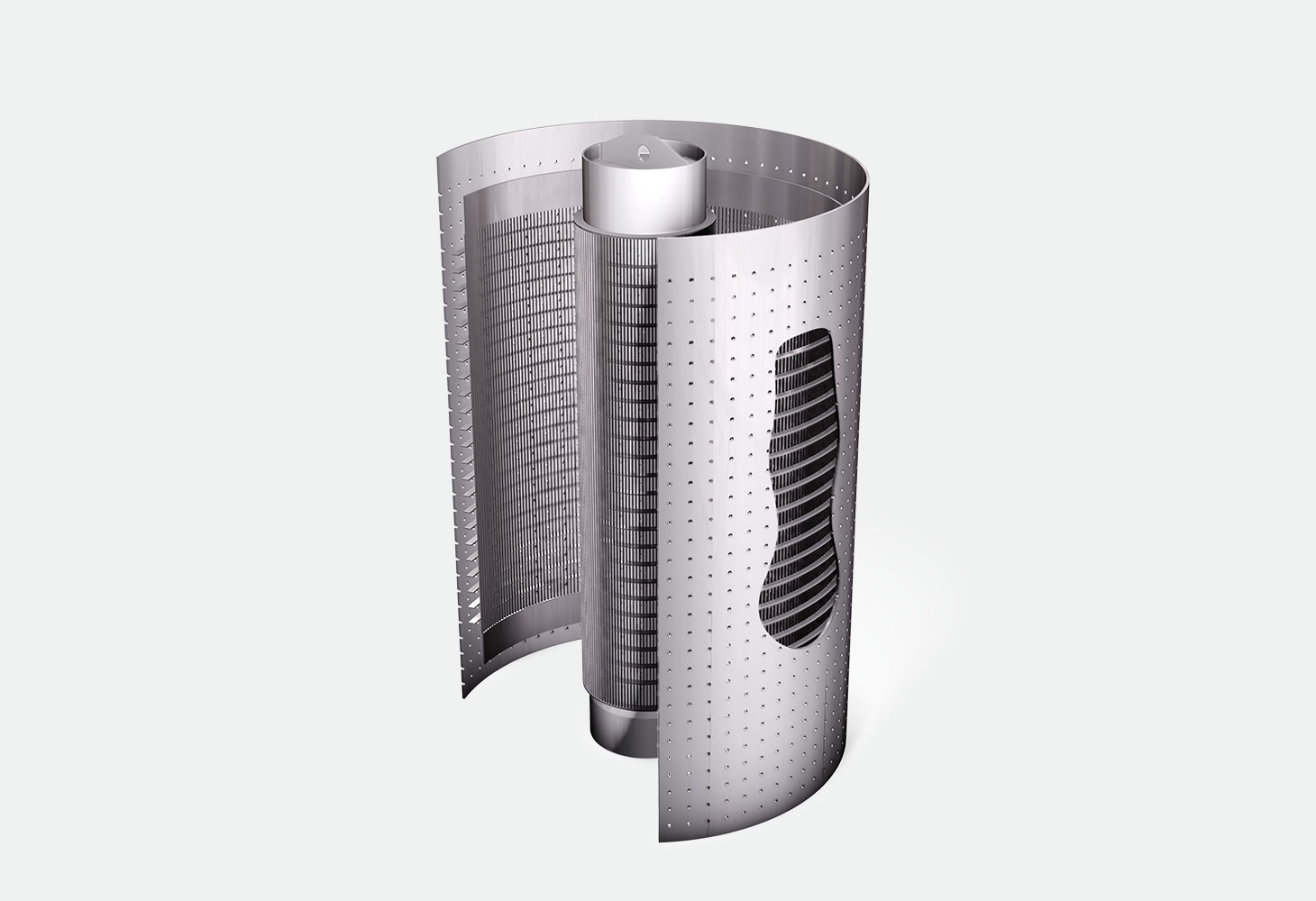
Ammonia synthesis reactor internals
The hydrogen and nitrogen mixture is compressed to the set pressure and is pre-heated by a heat exchanger counter current to the final product before entering the synthesis reactor.
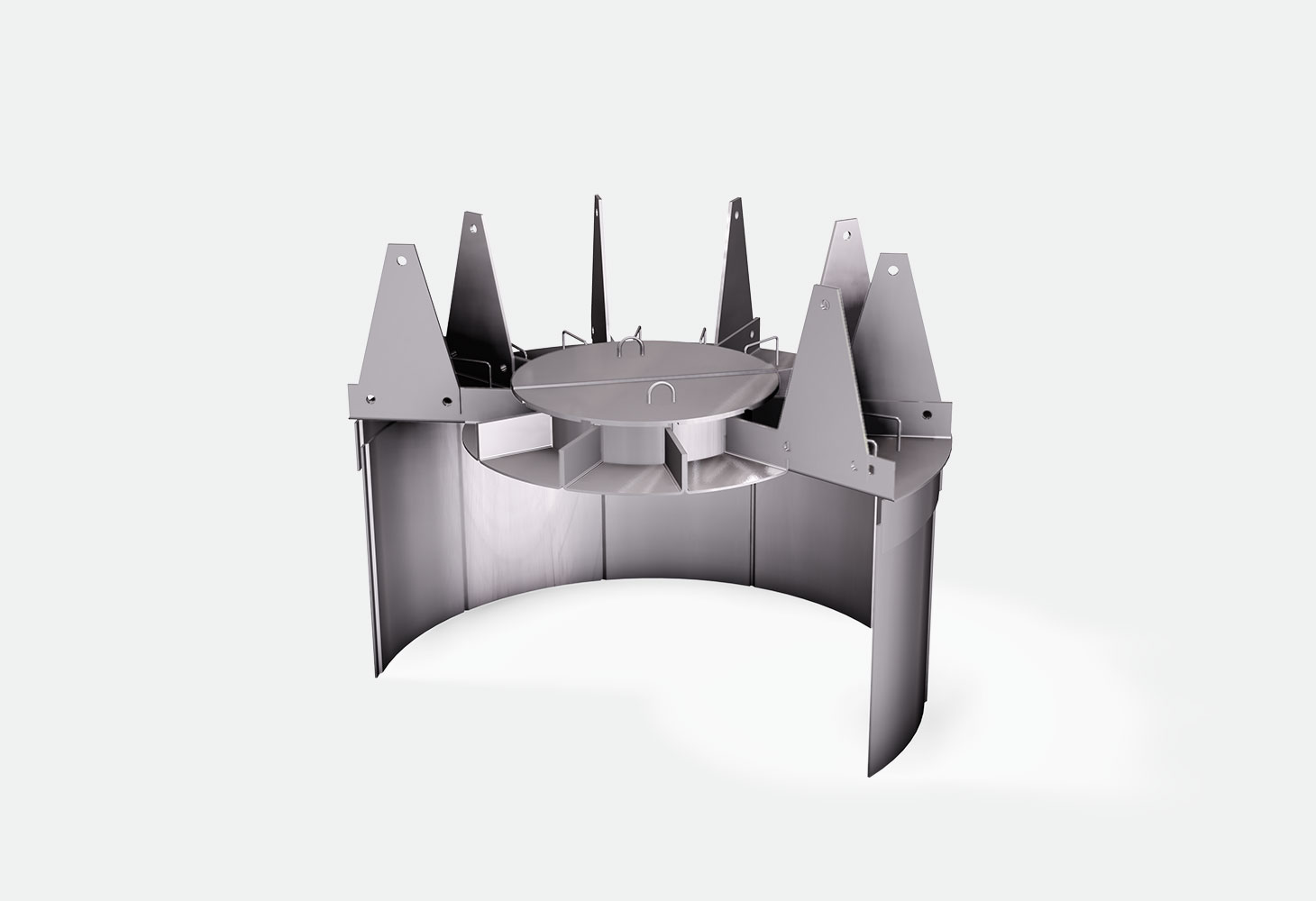
The ammonia synthesis reaction typically occurs in radial flow reactors over a catalytic bed at a high temperature. Internals, such as ‘outer baskets’ or ‘scallops’ allow the gas to be conveyed radially on the catalytic bed. After crossing the catalytic bed, the gas is collected in another internal, typically an ‘inner basket’. The outlet gas containing ammonia passes through a cooling chamber where the ammonia is condensed, while the unreacted hydrogen and nitrogen are recycled.
Distinctive characteristics of the Costacurta radial flow reactor internals for the conversion of ammonia are:
- Internal production of all the filtering elements used in making the internals (wire cloth weaving, punching and production of wedge wire screens)
- Internal production of all the internals, ‘scallops’, ‘outer’ and ‘inner baskets’, and the cartridges into which the internals are installed
- Over 60 years of experience in mechanical design and the production of internals
- Dedicated teams with long-standing experience in project management